Xanax Batch Recalled Nationwide Due To 'Potential Presence Of Foreign Substance'
Mylan Pharmaceuticals is pulling one lot of Xanax from pharmacy shelves nationwide because of what the company said is "the potential presence of a foreign substance" in the popular prescription anti-anxiety drug.Monday, October 28th 2019, 11:10 am
Mylan Pharmaceuticals is pulling one lot of Xanax from pharmacy shelves nationwide because of what the company said is "the potential presence of a foreign substance" in the popular prescription anti-anxiety drug.
The drug manufacturer cited a small chance of infection in recalling the batch of Alprazolam, the prescription drug sold under the brand name Xanax, according to a recall notice posted Saturday by the U.S. Food and Drug Administration.
"Clinical impact from the foreign material, if present, is expected to be rare, but the remote risk of infection to a patient cannot be ruled out," Mylan said.
The company did not immediately respond to requests for clarification on the foreign matter.
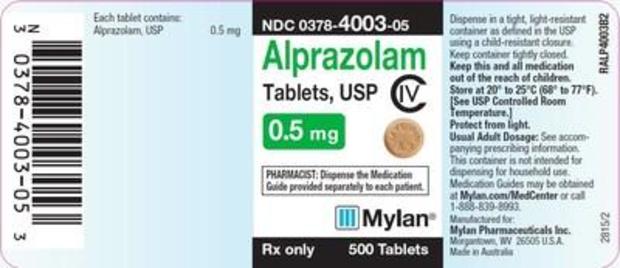
The recall involves Alprazolam Tablets, USP C-IV, 0.5 mg in 500-count bottles, lot No. 8082708, with a September 2020 expiration date. The lot was distributed in July and August.
Consumers who want to return the product can call (888) 843-0255. Consumers with questions about the recall can call Mylan at (800_ 796-9526 (Monday through Friday, 8 a.m. to 5 p.m., Eastern time) or by emailing customer.service@mylan.com.
More Like This
November 13th, 2024
October 28th, 2024
October 17th, 2024
Top Headlines
December 25th, 2024
December 25th, 2024
December 25th, 2024