The Race To A COVID-19 Vaccine
As we mask up and monitor the daily COVID trends, the only real hope for normalcy may be a vaccine. There are promising developments on the horizon and Oklahomans are playing a big part. News 9's Kelly Ogle has the story.Thursday, September 10th 2020, 10:48 pm
As we mask up and monitor the daily COVID trends, the only real hope for normalcy may be a vaccine. There are promising developments on the horizon and Oklahomans are playing a big part.
Lauren Murphey, an Oklahoma pharmacist, is going about her daily life knowing that she may have already received what so many people worldwide are desperately waiting for: a COVID-19 vaccine.
“My job is to kind of just go about as I have been,” she explained. “Which does include being careful, my social distancing, my mask wearing, all of that and then let them gather their data.”
She's part of a phase 3 study for the Moderna vaccine, one of the front runners. She agreed to be a part of the study and received her first injection on August 4.
“My arm was a little bit sore the day of the injection. But by the next day it felt totally fine. You can’t even really see any signs that I got it.”
Since it's a double-blind study Lauren doesn't know if she received the vaccine or a placebo. On August 31 she went back in for an antibody test and a second injection. Using an app on her phone she fills out daily questions about what she is experiencing. The U.S. Government recently agreed to pay Moderna to manufacture 100 million doses of the vaccine right now so it would be immediately available if the vaccine receives FDA approval. Possibly around the end of the year.
News 9’s Storme Jones is also part of a clinical trial for a different vaccine, but one of the three frontrunners. He got his first injection about a week and a half ago and reports no side effects.
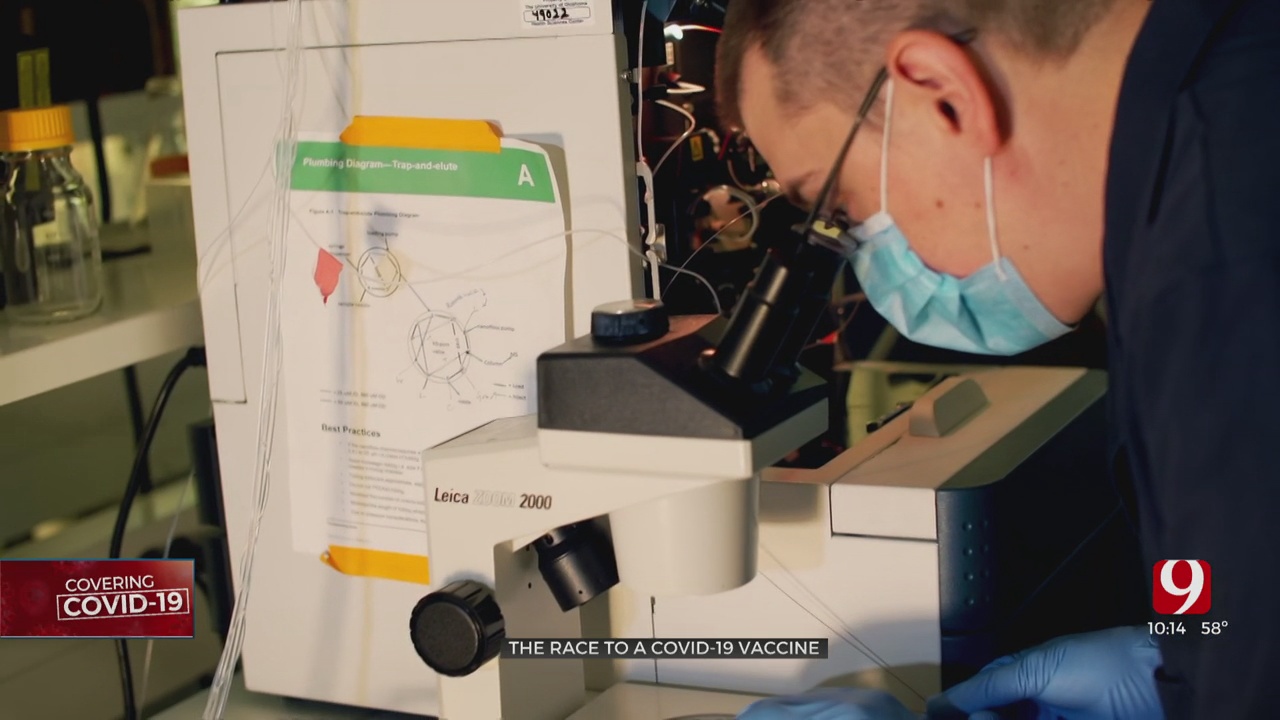
Meanwhile in a lab at OU Health Sciences Center, Dr. William Hildebrand and his team are working on a potential vaccine in case the Moderna or any of the others now in phase 3 trials don't work or aren't the best option.
“We really started planning the project in February when it was still quite controversial as to if this virus was going to be a problem or not,” he recalled.
Instead of focusing on a vaccine that extracts an antibody response. Hildebrand is looking at a vaccine that focuses on the body's other line of defense: T-Cells.
“We’re going to have a pretty good idea the fall and winter of this year 2020 how the antibody elicit vaccine is working, Moderna, Pfizer, AstraZeneca. Then we’ll be positioned if a second generation is needed or improvements or augmentation is needed to the antibody illicit vaccines, we’ll be able to jump into the game in 2021,” said Hildebrand.
They have already learned how the immune system detects COVID-19. Now they're trying to figure out how the body fights it off.
“A crew of maybe 10 to 15 people began working really long hours to get where we’re at right now,” said Hildebrand.
And they could have their vaccine ready for the public by the end of 2021.
“Based on history it would be the logical assumption that the first generation won’t be the last generation for a vaccine.”
Not far from Dr. Hildebrand's lab Lauren also does COVID testing as part of her job as a pharmacist. It puts her at a higher risk of getting the virus but also makes her a good candidate to see if a vaccine works.
And like those in that nearby lab she says she’s honored to be able to help try and end the pandemic.
“Just the chance to be a part of the solution and jump in there,” said Murphey.
Drug companies are still looking for paid participants for COVID vaccine trials. Click here for more information.
More Like This
September 10th, 2020
March 8th, 2022
January 21st, 2022
January 13th, 2022
Top Headlines
December 19th, 2024
December 19th, 2024
December 19th, 2024
December 19th, 2024