Oklahoma Doctors Celebrate Potential Breakthrough In Brain Cancer Treatment
Doctors at the Oklahoma Medical Research Foundation are celebrating what could become a breakthrough in brain cancer treatment. News 9's Karl Torp has the details.Wednesday, September 16th 2020, 5:56 pm
OKLAHOMA CITY -
Doctors at the Oklahoma Medical Research Foundation are celebrating what could become a breakthrough in brain cancer treatment.
It's a drug that could become the only answer for those facing the devastating diagnosis.
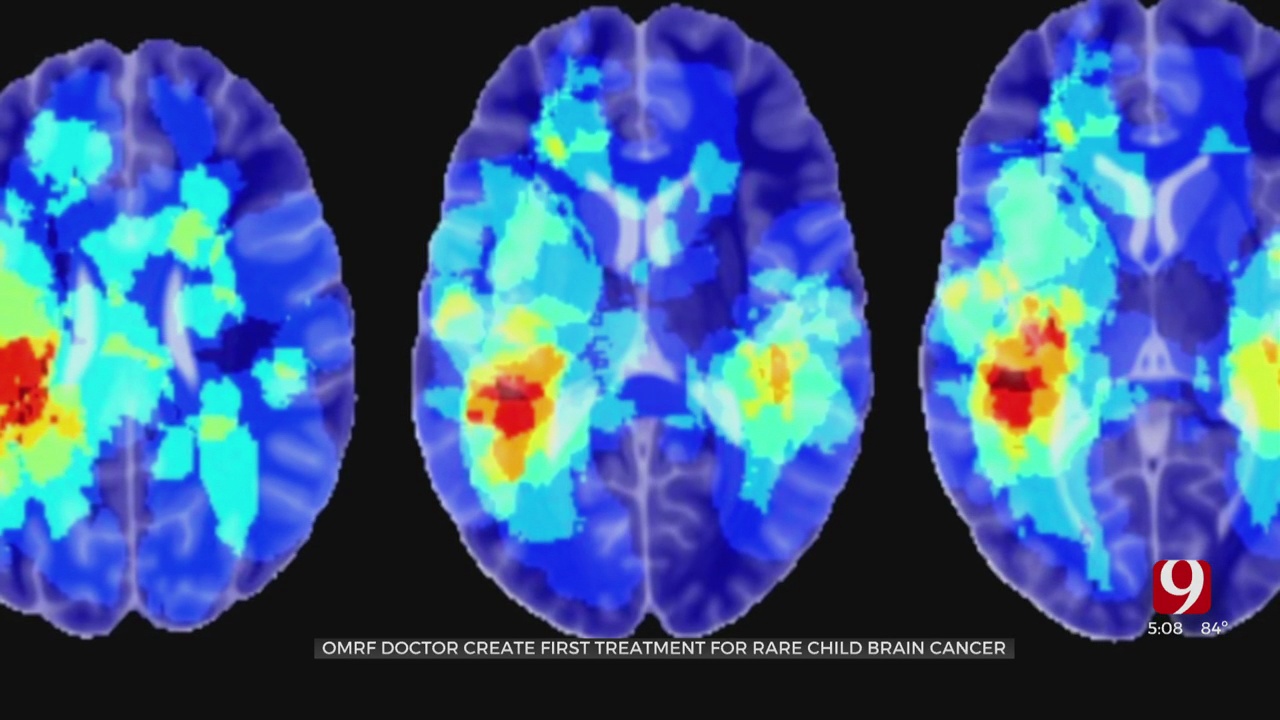
Researcher Dr. Rheal Towner has designed a medicine that fights DIPG, an uncurable form of pediatric brain cancer that effects around 300 children in the U.S. annually.
It took Dr. Towner around 30 years of research to come up with the medicine.
“This drug is actually really good and inhibiting tumor development in what we initially discovered was brain tumors,” said Towner.
The drug is called OKN-007.
It's now been FDA approved for clinical trials in hospitals and is expected to start early next year.
Its primary use was to treat glioblastoma in adults, but results showed promise in younger patients as well.
“We were surprised that it had much more of an affect than we anticipated,” added Towner.
If approved, OKN-007 would become the only FDA treatment for DIPG.
"Our drug is going to provide hope," said Towner.
More Like This
September 16th, 2020
April 16th, 2025
April 14th, 2025
April 11th, 2025
Top Headlines
April 17th, 2025
April 17th, 2025
April 17th, 2025
April 17th, 2025